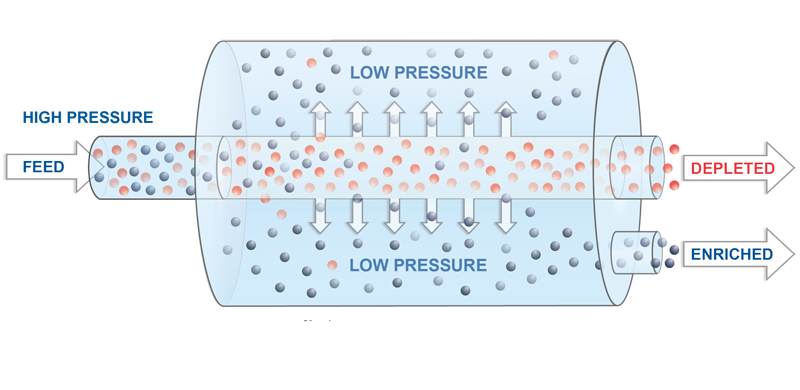
Gaseous diffusion uses uranium hexafluoride (UF6) as a feed material. UF6 is a solid at room temperature but becomes a gas when heated above 135 degrees Fahrenheit. Once heated to a gaseous state, the UF6 is fed into the plant’s cascades to be enriched.
The process separates the lighter U235 isotopes from the heavier U238. The gas is forced through a series of porous membranes with microscopic openings. Because the U235 is lighter, it moves through the barriers more easily.
As the gas moves, the two isotopes are separated, increasing the U235 concentration and decreasing the concentration of U238.
